The Institute of Vaccines and Medical Biologicals (IVAC), Nha Trang City has just excellently conducted a scientific study on seasonal influenza vaccine products...
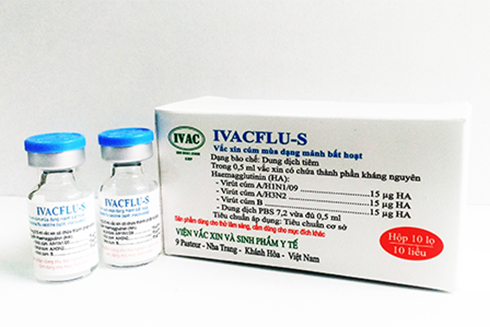
The Institute of Vaccines and Medical Biologicals (IVAC), Nha Trang City has just excellently conducted a scientific study on seasonal influenza vaccine products. The seasonal influenza vaccine named IVACFLU-S which targets 3 strains of influenza is expected to be produced and available on the market soon.
Duong Huu Thai, Vice-Director of IVAC says in 2007, the World Health Organization (WHO) provided aid for IVAC to build a WHO-GMP standard workshop, get technological transfer in terms of human training to construct a modern production line with a capacity of 1.5-3 million doses of influenza vaccine A/H5N1 a year. Thanks to the financial and technological assistance by the WHO and PATH (the USA), IVAC has successfully researched and produced A/H1N1/09, A/H5N1 and A/H7N9 vaccines. In addition, IVAC has studied and successfully developed the seasonal influenza 3-in-1 vaccine IVACFLU-S in Vietnam.
In 2012, IVAC started to build the 2nd generation seasonal influenza vaccine production technology. In 2013, approved by the Ministry of Health, IVAC used master seeds provided by the WHO though NIBSC (U.K) for vaccine production.
|
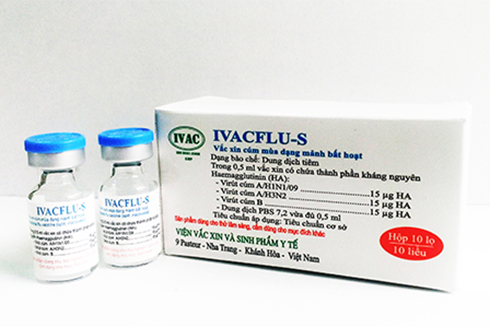
The pre-clinical test results on animals in labs proved the product safety and immunogenicity. Since 2015, the National Institute of Hygiene & Epidemiology and Ho Chi Minh Pasteur Institute have conducted a clinical test (3 phases) on humans. The research was examined and approved in May 2018. The IVACFLU-S vaccine produced by IVAC was reported to ensure quality, safety and immunogenicity, according to WHO and Vietnam regulations.
The IVACFLU-S vaccine technological production and clinical trial process is nearing completion. If approved, the vaccine will be launched on the market with a capacity of 1-1.5 million of doses/ year in early 2019. The vaccine is VND80,000-100,000/dose, saving 1/3 -1/2 off compared with imported ones, according to Duong Huu Thai.
Under the IVAC development plan, the vaccine will be accessible on the market and it will then be proposed by the Ministry of Health to be included in the open vaccination programs for those at high risk of influenza infection such as old people, children, pregnant women ... At the same time, IVAC will continue doing research to produce the third-generation influenza vaccines or vaccines against four strains; cooperate with WHO to further develop vaccines to cope with new variants of influenza viruses.
The IVAC’s successful manufacturing of the seasonal influenza 3-in-1 vaccine not only suits the Global Action Plan for Influenza Vaccines (GAP) launched by WHO but also facilitates more access to international standard influenza vaccines at discount prices.
T.Ly
Translated by N.T










